
Greatcare, a professional manufacturer with 22 years of expertise in the medical device industry, offers high-quality Combined Spinal and Epidural Anaesthesia Kits. These kits are manufactured under strict quality standards, certified by CE and ISO13485. With approvals including China and Europe free sale certificates, they provide a reliable and cost-effective solution for anesthesia needs.
Product Introduction
The Epidural Anaesthesia Kit consists of epidural needle, spinal needle, epidural catheter (general type or reinforced type), catheter connector, introducer guide, liquid filter, low-resistance syringe. It is used for spinal and epidural puncture, and injection of liquid drugs into the epidural space and the subarachnoid space during epidural anesthesia and spinal anesthesia.
Product Specification
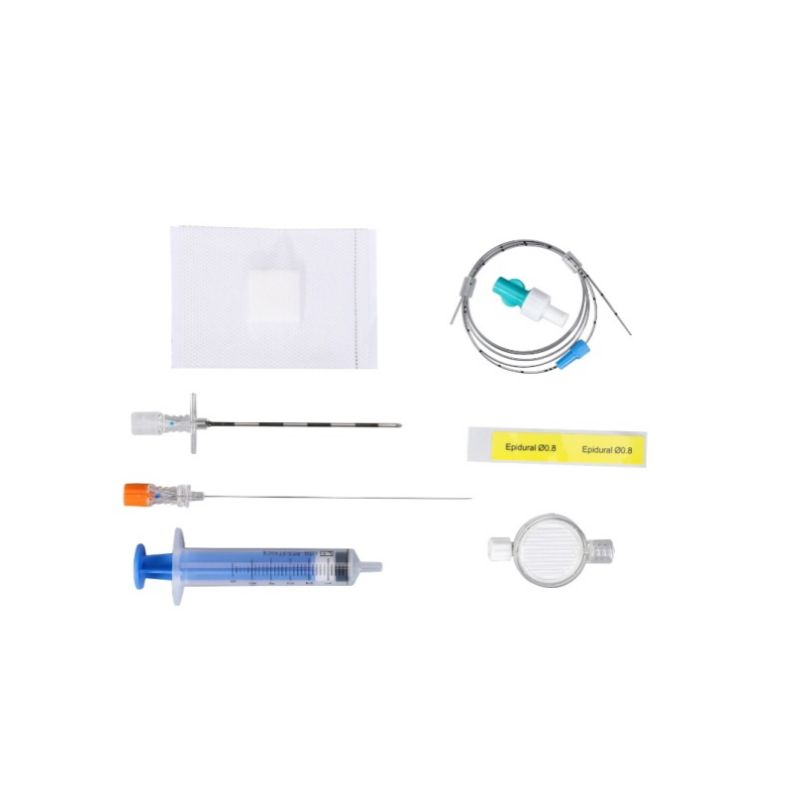
| No. | Components List | Specification |
| 1 | Eqidural needle (tuohy needle) | 16/18G*80mm |
| 2 | Spinal needle | 25G*110mm, pencil point |
| 3 | Eqidural catheter | Multiport with mark 0.8/1.0mm, length≥850mm |
| 4 |
Epidural filter |
0.22/0.2μm, hydrophobic membrane, Luer lock |
| 5 |
LOR syringe |
Both 5ml, 7ml and 10ml specifications are available. |
| 6 |
Catheter adaptor |
/ |
| 7 |
Wound dressing |
/ |
1. Single-use, sterile packaging to prevent cross-contamination.
2. Complete accessory configuration to reduce preparation time in the operating room.
3. Clear and accurate depth markings for easy observation of catheter insertion length.
Directions for use
● First inspect the integrity of the package, and then remove product from the sterile package, and inspect the integrity of product.
● Properly disinfect the puncture site before local anesthesia.
● A common method is to apply epidural puncture. Connect low resistance syringe to epidural needle seat and use negative pressure test to detect whether or not epidural needle enters epidural space. Stylet blade should be withdrawn before contact with ligamentum flavum. Operator can position needle through needle markings. ligamentum flavum. Operator can position needle through needle markings.
● Carefully insert the spinal needle through the epidural needle until resistance is encountered at the needle tip. Carefully insert the spinal needle into the subarachnoid space, and observe if there is cerebrospinal fluid flow out to determine whether or notspinal needle has entered the subarachnoid space.
● For successful puncture, please fix the puncture site and connect the spinal needle to low-resistance syringe. Before injection, stylet should be withdrawn and to observe if there is cerebrospinal fluid flow out. If an outflow of cerebrospinal fluid is observed, it proves that the needle has reached the subarachnoid space.
● The syringe (containing anesthetic liquid) is connected with the inlet end of the liquid filter, and the outlet end of the liquid filter is connected with the needle hub of spinal needle. The liquid anesthetic is injected according to the diffusion rate of the liquid medicine, and the patient is observed and monitored according to the requirements of spinal anesthesia.
● Spinal needle should be withdrawn when injection is completed.
● The tip of the epidural catheter (general type or reinforced type) penetrates into the epidural needle lumen through the introducer guide, enters to the epidural space 30-50mm, and then withdraws the epidural needle slowly.
● The inlet end of the epidural catheter (general type or reinforced type) is connected to the catheter connector, the catheter connector is connected to the outlet end of the liquid filter, and the liquid filter is connected to a syringe containing the liquid anesthetic, and the drug is administered as required by the operation.
● In general, the epidural catheter (general type or reinforced type) can be withdrawn after the completion of surgery. If postoperative analgesia is needed, it can be connected with analgesic devices, and the epidural catheter (general type or reinforced type) can be withdrawn after analgesia is completed.
FAQ
Q: What is the delivery time if I place my order?
A: Delivery time is around 45 days, if you have special requirements, pls check with us, we will try our best to meet you.
Q: Can you supply the relevant documentation?
A: Yes, we can provide most documentation including CE, ISO13485, FSC, FDA where required.
Q: Can I get samples before my order ?
A: Free samples are available.